If Someone Has Hypogonadism Gonad Ism Can They Have Babies
Introduction
Testosterone (T) therapy has garnered widespread public enthusiasm and media attention due to its potential role in age-related T decline in men, commonly known every bit late-onset hypogonadism (LOH), andropause, or depression T syndrome. The serum T concentration gradually declines beyond the lifespan and the symptoms between aging and hypogonadism overlap. These have led to the speculation that a causal relationship might exists between age-related reduction in serum T concentration and symptoms commonly seen in aging. However, it remains uncertain if T therapy could meliorate symptoms associated with LOH, without significant risks. Despite the lack of clinical evidence and long term rubber data, prescribing rates of T therapy accept skyrocketed in many countries (1, two), leading to efforts by regulatory government to limit such inappropriate prescribing practice (3).
Importantly, the primal question of what constitutes clinically significant LOH was largely unaddressed until recently. Heterogeneity in definitions of LOH and the use of specificity-limited immunoassays for T measurements in many previous epidemiological and interventional studies have precluded robust comparisons across studies (4). Due to the expanding aging population, LOH is becoming an increasingly important topic. We reviewed the bear witness from contempo population-based studies and intervention trials to provide better understanding of the diagnosis, pathophysiology, and management for LOH.
Pathophysiology of T Reject in Aging
The testicular function undergoes natural pass up with age. Compared to younger men, good for you older men has 40% less Leydig prison cell mass and a corresponding rising in luteinizing hormone (LH) concentration (5). Decreased testicular T production was also observed in aged Leydig cells, following diminished LH-stimulated cAMP production, and reduced downstream steroidogenic enzymatic activity (6). On the other hand, aging is associated with changes in LH secretory design. A reduced T product and frequent, small irregular LH pulses was observed in healthy older men (7), despite preservation of pituitary gonadotrophs' response to exogenous gonadotropin-releasing hormone (GnRH) (viii).This suggests age or factors associated with aging reduced negative feedback inhibition by T. An ensemble-based assay also predicted a >30% fall in GnRH output in healthy older men (nine). Nevertheless, a contempo study has demonstrated that healthy older men without belatedly-onset hypo-gonadism (LOH) take preserved hypothalamic response to kisspeptin-54 and pituitary response to GnRH, with impaired testicular response equally compared to younger men (10). This suggests that primary testicular failure accounts principally for the normal crumbling-related pass up in T production. In majority of healthy older men, the compensatory increase in gonadotrophins serves to maintain T levels inside eugonadal ranges (11).
The pathophysiology of LOH is complicated by comorbidities associated with aging. The development of chronic illnesses, including diabetes, cardiovascular affliction and inflammatory disorders, is associated with a contemporaneous accelerated rate of aging-related T decline, ranging between one.five- and 3.6-fold compared to men who remain illness-free (12, 13). Furthermore, backlog adiposity exerts potent suppressive effects on the HPT axis. Individuals with BMI ≥30 kg/mtwo are at 13-fold increased adventure of LOH compared to those with BMI <25 kg/one thousand2 (14). Overall, men with comorbidity and/or obesity failed to showroom compensatory rising in LH levels which would otherwise expected in salubrious not-obese men suggesting a significant disruption at the hypothalamic-pituitary level which compromises T production (15).
Consonant with that, obesity has been shown to exist the well-nigh common cistron associated with the development of low T in centre-anile and older men (xi). The pathogenic role of excess adiposity has been postulated to be linked to several adipose tissue-derived factors, including pro-inflammatory cytokines and leptin, and contradistinct insulin-signaling, which deed in concert to produce central inhibitory furnishings on the HPT axis, leading to secondary hypogonadism (16–xviii). Interesting, obesity likewise increase oxidative/nitrosative stress leading to nitroso-redox imbalance and male sexual dysfunction (19). The potential mechanisms underpin the development of LOH is depicted in Effigy ane.
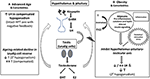
Effigy 1. Mechanistic caption for low serum T in middle-aged and older men. (A) As Leydig jail cell reserve decline with aging, compensatory ascension in luteinising hormone (LH) occurs to maintain circulating testosterone (T) concentrations (compensated hypogonadism). In more than advanced state, elevated LH can no longer overcome the macerated testicular function, leading to overtly low T levels (primary hypogonadism). (B) Obesity is the predominant crusade of functional suppression of hypothalamic-pituitary-testicular (HPT) axis in middle-anile and older men, manifesting equally failure of LH response to depression T (secondary hypogonadism). Multimorbidity is also associated with both primary and secondary hypogonadism, albeit to a bottom degree. Excess adiposity has been linked to altered insulin signaling, oxidative stress and increased pro-inflammatory cytokines and leptin levels, which act in concert to suppress the primal HPT centrality. Adipose tissues also express aromatase which convert testosterone to estradiol, especially in the inflammed state, exerting inhibitory furnishings on the HPT axis.
AGING-related Decline in T Concentrations
Serum total T concentrations were historically idea to turn down at a charge per unit of 1–two% per annum from 4 to 5th decade onwards (20, 21). One of the population-based studies demonstrated that >50% of men anile ≥80 years had T level in hypogonadal range, as defined by <ii.5th percentile for young men (<11.3 nmol/50) (22).
However, accumulating evidence from newer studies suggest that age-related autumn in serum total T is closer to 0.five% per twelvemonth (12, fifteen, 23), and healthy older men actually feel minimal changes in T levels. A community-based longitudinal study from South Australia showed that the rate of decline of full T concentrations in a subset of men without chronic illnesses was a non-significant 0.27% per year (12). In another report, no appreciable change in serum T up to 8th decade of life was observed among men with self-reported very adept to excellent health (24). In European Male Aging Study (EMAS), two,736 men aged ≥40 years were followed up for an average of 4.4 years, and >80% of men in their 7–8th decade continued to have normal T values (11). Therefore, LOH is less prevalent than previously idea, and low T in older men is mostly related to co-existing medical weather condition and obesity.
The Challenges in Diagnosing LOH
LOH has conventionally been defined as depression serum T in older men, irrespective of the luteinizing hormone (LH) levels. This has led to a prevalence as high as 50% been quoted in some studies. However, the European Male Aging Written report (EMAS) has demonstrated 2 singled-out groups of older men with low total T (14). The bulk of older men were found to accept low T associated with low-normal luteinizing hormone. This is not independently associated with aging per se but is mediated indirectly via age-related non-gonadal co-morbidities, including obesity, and increased visceral adiposity. Merely a pocket-sized number of older men (ii.i%) had low T with loftier LH, in keeping with principal testicular insufficiency. This specific primary hypogonadism profile has been direct associated with both aging and metrics of sick wellness.
On the other hand, it is imperative that the diagnostic evaluation of male person hypogonadism be corroborated with signs and symptoms (25). However, there is substantial overlap between symptoms arising from chronic diseases and hypogonadism, posing significant challenge to determining clinically relevant LOH (14). Indeed, men reported hypogonadal symptoms frequently accept T concentrations in the eugondal ranges (26). Moreover, the clinical significance of borderline or modestly low T levels typically seen in LOH is often hard to define.
To accost some of these gaps, EMAS investigators established a gear up of minimum criteria (xiv). In this report, 32 candidate symptoms were shortlisted, and after reductive analysis, merely the co-occurrence of 3 sexual symptoms (decreased morning erection, poor libido, erectile dysfunction) and low T level (total T < xi nmol/L and complimentary T < 220 pmol/L) had consequent syndromic association. With that, the overall prevalence of LOH in EMAS population was determined to be 2.1%, widely believed to be the most accurate approximate hitherto, lower than previous studies using less stringent criteria (26). Stratifying by age groups, <1% of men aged <sixty years, 3.2% of men anile threescore–69 years, and 5.one% of men anile seventy–79 years met the proposed criteria.
The nomenclature of LOH Co-ordinate to LH Level and Associated Risk Factors
The hypothalamus-pituitary- testicular (HPT) centrality is tightly regulated in an interdependent fashion to maintain hormonal homeostasis. In hypogonadism, the gonadotropins can either exist elevated (primary hypogonadism) or low/normal (secondary hypogonadism). In EMAS, subjects are classified into primary hypogonadism (LH > 9.four u/L, T < x.5 nmol/L), secondary hypogonadism (LH ≤ 9.4 u/50, T < 10.v nmol/L) or compensated (principal) hypogonadism (LH > 9.4 u/L, T ≥ x.v nmol/L) (11). Through this arroyo, unique clinical characteristics and risk factors were identified in each subgroup.
Primary hypogonadism was found to be uncommon in the study. It afflicted only 2% of the entire cohort and had a depression almanac incidence of 0.two% (27). At-hazard men had poorer baseline physical function, and suffered from deterioration in erectile function, vigor and hemoglobin as they progressed to hypogonadism. Advanced age (>70 years) and comorbidities were strongly associated with increased take chances of chief hypogonadism, with an odds ratio of 12.5 and 4.24, respectively. The serum T concentrations connected to turn down with time with piddling sign of recovery. For the minority whom T levels returned to eugonadal range, the mean LH levels remained persistently elevated to the same degree, indicating persistent Leydig prison cell failure.
Secondary hypogonadism deemed for majority (85.five%) of older men with low T, with an annual incidence of ane.vi% (11). The mean LH level was not different from that of eugonadal men, indicating a failure in the compensatory hypothalamic-pituitary centrality. Different primary hypogonadism, in that location was no significant human relationship betwixt the prevalence of secondary hypogonadism and aging. Instead, obesity emerged to be the most potent risk factor (14, 15), with a bottom contribution by comorbidities. Therefore, secondary hypogonadism represents a state of functional HPT suppression driven principally by obesity and poor health, rather than chronological aging.
The third classification was compensated hypogonadism, present in close to 10% of the study cohort. This grouping of men had normal circulating total T concentration and raised LH level. They exhibited some clinical features in keeping with principal hypogonadism (27), making it a clinically relevant entity. Despite being relatively common, progression to hypogonadism range of T concentration was very infrequent, suggesting that most men in this group could retain the capacity to sustain adequate T levels.
Management of LOH
Subtyping LOH according to both T and LH levels provides useful clinical data in elucidating the underlying etiology, and allows management to be tailored appropriately. For LOH due to testicular failure (master hypogoadism), T treatment could exist used to improve anemia, sexual practice and libido in older men (28–34). All the same, T therapy was found to take no pregnant impact on free energy level, physical part, weight, or cognitive function among older men with LOH (28, 35–40). Despite the reassuring data from majority of interventional trials with regards to short term safe (41–44), a meta-assay of 27 placebo-controlled trials has ended that T therapy was associated with an increased cardiovascular gamble, with an odds ratio of 1.54 (95% confidence interval, i.09 to ii.xviii) (45). Furthermore, T therapy is associated with increased hematocrit, serum concentrations of prostate-specific antigen (PSA) and prostate book, as well equally gynecomastia and secondary infertility. Hence, T therapy should only be considered subsequently conscientious consideration of the risks and benefits, while begetting in mind that the cardiovascular safety profile of T therapy in this population has yet to be fully established. Ongoing surveillance of hematocrit and prostate specific antigen is also required whilst on T treatment (24).
On the other mitt, human chorionic gonadotropin (HCG) may accept a therapeutic part in LOH (46, 47). HCG therapy is known to increase serum testosterone concentration and preserve global action of the testis (east.thousand., fertility and insulin-like factor three production) (48, 49). A clinical trial comparing vi-months HCG vs. T therapy in LOH has demonstrated higher 25-OH-vitamin D and lower serum estradiol concentrations in men treated with HCG (47). The prostate book and hematocrit level were too significantly lower compared to the groups treated with T (47). The Leydig cells have been shown to contribute to the 25-hydroxylation of vitamin D and a higher 25-OH-vitamin D level may reflect improved Leydig cell function following HCG handling (l). Hence, HCG therapy may have a favorable contour in LOH but larger safety and efficacy trials would be required to decide if HCG could exist used equally a long-term therapy in LOH.
It should be emphasized that obesity and co-morbidities underlies near cases of depression T in older men with secondray hypogonadism, and thus, lifestyle intervention and cardiometabolic risk reduction should be the first line treatment for this cohort of patients. Notably, the potential for reversal to eugonadism in secondary hypogonadism is promising for obese men; nearly one-half of the men recovered their T levels over a period of ~iv years, predicted past attainment of healthier weight (51).
Determination
Establishing the diagnosis of LOH remains a conundrum in clinical exercise because of imprecise criteria and confounding factors relating to health alterations in old age. Withal, if we ascertain LOH as age-related primary testicular failure, only a minority of men appears to be affected. While studies have demonstrated some positive effects of T therapy, the clinical meaningfulness of these findings remains debatable. Moreover, the absence of long-term cardiovascular rubber data continues to be an area of business and controversy.
Hence, we advise that future interventional trials for LOH should aim at older men with principal testicular failure, or classify the study cohorts according to LH levels and so that a more clinically meaningful take a chance-benefit stratification can be elicited. This will clarify the safety and do good contour of T therapy or other treatments in LOH and inform decision of the about appropriate management for LOH in men.
Writer Contributions
DS wrote the first draft of the newspaper. EG amended and rewrote the paper so that information technology matches the opinion style (paper was showtime submitted as review). EG produced the figure.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of whatever commercial or financial relationships that could be construed as a potential disharmonize of involvement.
References
1. Liverman CT, Blazer DG editors. Testosterone and Aging:Clinical Enquiry Directions. Washington, DC: National Academics Printing (2004).
Google Scholar
3. Nguyen CP, Hirsch MS, Moeny D, Kaul Due south, Mohamoud M, Joffe HV. Testosterone and "Age-related hypogonadism" — FDA concerns. N Engl J Med. (2015) 373:689–91. doi: x.1056/NEJMp1506632
PubMed Abstract | CrossRef Full Text | Google Scholar
iv. Handelsman DJ, Wartofsky Fifty. Requirement for mass spectrometry sex steroid assays in the journal of clinical endocrinology and metabolism. J Clin Endocrinol Metab. (2013) 98:3971–three. doi: 10.1210/jc.2013-3375
PubMed Abstruse | CrossRef Total Text | Google Scholar
v. Neaves WB, Johnson L, Porter JC, Parker CR, Trivial CS. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab. (1984) 59:756–63. doi: 10.1210/jcem-59-4-756
PubMed Abstract | CrossRef Full Text | Google Scholar
seven. Liu PY, Takahashi PY, Roebuck PD, Veldhuis JD. Age or factors associated with crumbling benumb testosterone's concentration-dependent enhancement of the regularity of luteinizing hormone secretion in healthy men. J Clin Endocrinol Metab. (2006) 91:4077–84. doi: 10.1210/jc.2005-2811
CrossRef Full Text | Google Scholar
8. Mulligan T, Iranmanesh A, Kerzner R, Demers LW, Veldhuis JD. Two-calendar week pulsatile gonadotropin releasing hormone infusion unmasks dual (hypothalamic and Leydig cell) defects in the healthy aging male person gonadotropic axis. Eur J Endocrinol. (1999) 141:257–66. doi: x.1530/eje.0.1410257
PubMed Abstract | CrossRef Full Text | Google Scholar
ix. Keenan DM, Takahashi PY, Liu PY, Roebuck PD, Nehra AX, Iranmanesh A, et al. An ensemble model of the male gonadal axis: Illustrative application in aging men. Endocrinology. (2006) 147:2817–28. doi: ten.1210/en.2005-1356
PubMed Abstract | CrossRef Full Text | Google Scholar
ten. Abbara A, Narayanaswamy S, Izzi-Engbeaya C, Comninos AN, Clarke SA, Malik Z, et al. Hypothalamic response to kisspeptin-54 and pituitary response to gonadotropin-releasing hormone are preserved in healthy older men. Neuroendocrinology. (2018) 106:401–10. doi: 10.1159/000488452
PubMed Abstract | CrossRef Full Text | Google Scholar
xi. Tajar A, Forti 1000, O'Neill TW, Lee DM, Silman AJ, Finn JD, et al. Characteristics of principal, secondary and compensated hypogonadism in ageing men: evidence from the European Male person Ageing Study (EMAS). J Clin Endocrinol Metab. (2010) 95:1810–8. doi: x.1210/jc.2009-1796
CrossRef Total Text | Google Scholar
12. Shi Z, Araujo AB, Martin S, O'Loughlin P, Wittert GA. Longitudinal changes in testosterone over five years in community-dwelling men. J Clin Endocrinol Metab. (2013) 98:3289–97. doi: x.1210/jc.2012-3842
PubMed Abstract | CrossRef Full Text | Google Scholar
xiii. Travison TG, Araujo AB, Kupelian V, O'Donnell AB, McKinlay JB. The relative contributions of crumbling, wellness, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab. (2007) 92:549–55. doi: 10.1210/jc.2006-1859
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. Identification of belatedly-onset hypogonadism in middle-aged and elderly men. N Engl J Med. (2010) 363:123–35. doi: ten.1056/NEJMoa0911101
PubMed Abstract | CrossRef Total Text | Google Scholar
xv. Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O'Neill TW, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European male aging study. J Clin Endocrinol Metab. (2008) 93:2737–45. doi: 10.1210/jc.2007-1972
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Gautier A, Bonnet F, Dubois Due south, Massart C, Grosheny C, Bachelot A, et al. Associations between visceral adipose tissue, inflammation and sexual practice steroid concentrations in men. Clin Endocrinol. (2013) 78:373–viii. doi: 10.1111/j.1365-2265.2012.04401.ten
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Veldhuis J, Yang R, Roelfsema F, Takahashi P. Proinflammatory cytokine infusion attenuates lh's feedforward on testosterone secretion: modulation past age. J Clin Endocrinol Metab. (2016) 101:539–49. doi: 10.1210/jc.2015-3611
PubMed Abstract | CrossRef Full Text | Google Scholar
eighteen. Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert Thousand, Orban PC, et al. Role of brain insulin receptor in control of body weight and reproduction. Scientific discipline. (2000) 289:2122–v. doi: ten.1126/science.289.5487.2122
PubMed Abstruse | CrossRef Total Text | Google Scholar
19. Kuchakulla M, Masterson T, Arora H, Kulandavelu Southward, Ramasamy R. Outcome of nitroso-redox imbalance on male reproduction. Transl Androl urol. (2018) 7:968–77. doi: 10.21037/tau.2018.08.fourteen
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al. Age trends in the level of serum testosterone and other hormones in middle-anile men: longitudinal results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. (2002) 87:589–98. doi: 10.1210/jc.87.two.589
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Lapauw B, Goemaere Due south, Zmierczak H, Van Pottelbergh I, Mahmoud A, Taes Y, et al. The pass up of serum testosterone levels in community-dwelling house men over 70 years of age: descriptive data and predictors of longitudinal changes. Eur J Endocrinol. (2008) 159:459–68. doi: ten.1530/EJE-07-0873
PubMed Abstract | CrossRef Full Text | Google Scholar
22. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Aging BLS of. longitudinal furnishings of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Report of Aging. J Clin Endocrinol Metab. (2001) 86:724–31. doi: 10.1210/jc.86.2.724
CrossRef Full Text | Google Scholar
23. Handelsman DJ, Yeap B, Flicker L, Martin South, Wittert GA, Ly LP. Age-specific population centiles for androgen condition in men. Eur J Endocrinol. (2015) 173:809–17. doi: 10.1530/EJE-15-0380
PubMed Abstract | CrossRef Total Text | Google Scholar
24. Sartorius 1000, Spasevska S, Idan A, Turner Fifty, Forbes E, Zamojska A, et al. Serum testosterone, dihydrotestosterone and estradiol concentrations in older men cocky-reporting very good health: the healthy man written report. Clin Endocrinol. (2012) 77:755–63. doi: 10.1111/j.1365-2265.2012.04432.10
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Bhasin South, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, et al. Testosterone therapy in men with hypogonadism: an endocrine society clinical exercise guideline. J Clin Endocrinol Metab. (2018) 103:1715–44. doi: 10.1210/jc.2018-00229
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Araujo AB, Esche GR, Kupelian V, O'Donnell AB, Travison TG, Williams RE, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. (2007) 92:4241–7. doi: 10.1210/jc.2007-1245
PubMed Abstruse | CrossRef Full Text | Google Scholar
27. Ahern T, Swiecicka A, Eendebak RJ, Carter EL, Finn JD, Pye SR, et al. Natural history, risk factors and clinical features of primary hypogonadism in ageing men: longitudinal data from the European Male person Ageing Written report. Clin Endocrinol. (2016) 85:891–901. doi: 10.1111/cen.13152
PubMed Abstract | CrossRef Full Text | Google Scholar
28. Snyder PJ, Bhasin South, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, et al. Furnishings of testosterone treatment in older men. Northward Engl J Med. (2016) 374:611–24. doi: x.1056/NEJMoa1506119
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Wu F, Zitzmann 1000, Heiselman D, Donatucci C, Knorr J, Patel AB, et al. Demographic and clinical correlates of patient-reported comeback in sex bulldoze, erectile function, and energy with testosterone solution 2%. J Sex Med. (2016) 13:1212–ix. doi: 10.1016/j.jsxm.2016.05.010
PubMed Abstract | CrossRef Total Text | Google Scholar
30. Corona G, Isidori AM, Buvat J, Aversa A, Rastrelli 1000, Hackett Thou, et al. Testosterone supplementation and sexual function: a meta-analysis study. J Sex Med. (2014) xi:1577–92. doi: 10.1111/jsm.12536
PubMed Abstract | CrossRef Full Text | Google Scholar
31. Elliott J, Kelly SE, Millar Ac, Peterson J, Chen L, Johnston A, et al. Testosterone therapy in hypogonadal men: a systematic review and network meta-analysis. BMJ Open. (2017) 7:e015284. doi: x.1136/bmjopen-2016-015284
PubMed Abstract | CrossRef Full Text | Google Scholar
32. Huo S, Scialli AR, McGarvey Southward, Colina E, Tügertimur B, Hogenmiller A, et al. Handling of men for "low testosterone": a systematic review. PLoS ONE. (2016) 11:e0162480 doi: 10.1371/journal.pone.0162480
PubMed Abstract | CrossRef Full Text | Google Scholar
33. Roy CN, Snyder PJ, Stephens-Shields AJ, Artz Every bit, Bhasin Southward, Cohen HJ, et al. Association of testosterone levels with anemia in older men a controlled clinical trial. JAMA Intern Med. (2017) 177:480–xc. doi: 10.1001/jamainternmed.2016.9540
PubMed Abstract | CrossRef Full Text | Google Scholar
34. Traustadóttir T, Harman SM, Tsitouras P, Pencina KM, Li Z, Travison TG, et al. Long-term testosterone supplementation in older men attenuates age-related decline in aerobic capacity. J Clin Endocrinol Metab. (2018) 103:2861–9. doi: 10.1210/jc.2017-01902
PubMed Abstract | CrossRef Total Text | Google Scholar
35. Huan K, Pencina KM, Li Z, Basaria South, Bhasin Southward, Travison TG, et al. Long-term testosterone administration on insulin sensitivity in older men with low or low-normal testosterone levels. J Clin Endocrinol Metab. (2018) 103:1678–85. doi: ten.1210/jc.2017-02545
CrossRef Full Text | Google Scholar
36. Magnussen LV, Glintborg D, Hermann P, Hougaard DM, Højlund Chiliad, Andersen K. Effect of testosterone on insulin sensitivity, oxidative metabolism and body limerick in crumbling men with type 2 diabetes on metformin monotherapy. Diabetes Obes Metab. (2016) xviii:980–9. doi: x.1111/dom.12701
PubMed Abstract | CrossRef Full Text | Google Scholar
37. Huang 1000, Wharton Due west, Bhasin S, Harman SM, Pencina KM, Tsitouras P, et al. Effects of long-term testosterone assistants on cognition in older men with low or low-to-normal testosterone concentrations: a prespecified secondary analysis of data from the randomised, double-blind, placebo-controlled TEAAM trial. Lancet Diabetes Endocrinol. (2016) 4:657–65. doi: 10.1016/S2213-8587(sixteen)30102-4
PubMed Abstruse | CrossRef Full Text | Google Scholar
38. Resnick SM, Matsumoto AM, Stephens-Shields AJ, Ellenberg SS, Gill TM, Shumaker SA, et al. Testosterone treatment and cognitive function in older men with low testosterone and age-associated retentivity damage. JAMA. (2017) 317:717. doi: x.1001/jama.2016.21044
PubMed Abstract | CrossRef Total Text | Google Scholar
39. Travison TG, Basaria S, Storer TW, Jette AM, Miciek R, Farwell WR, et al. Clinical meaningfulness of the changes in muscle operation and physical function associated with testosterone administration in older men with mobility limitation. Journals Gerontol - Ser A Biol Sci Med Sci. (2011) 66 A:1090–9. doi: ten.1093/gerona/glr100
CrossRef Total Text | Google Scholar
xl. Storer TW, Basaria Due south, Traustadottir T, Harman SM, Pencina K, Li Z, et al. Furnishings of testosterone supplementation for 3 years on musculus performance and physical part in older men. J Clin Endocrinol Metab. (2017) 102:583–93. doi: 10.1210/jc.2016-2771
PubMed Abstract | CrossRef Full Text | Google Scholar
41. Corona G, Maseroli E, Rastrelli G, Isidori AM, Sforza A, Mannucci E, et al. Cardiovascular hazard associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf . (2014) 13:1327–51. doi: ten.1517/14740338.2014.950653
PubMed Abstract | CrossRef Full Text | Google Scholar
42. Budoff MJ, Ellenberg SS, Lewis CE, Mohler ER, Wenger NK, Bhasin Southward, et al. Testosterone treatment and coronary artery plaque book in older men with depression testosterone. JAMA. (2017) 317:708–16. doi: ten.1001/jama.2016.21043
PubMed Abstract | CrossRef Full Text | Google Scholar
43. Alexander GC, Iyer Thousand, Lucas Due east, Lin D, Singh S. Cardiovascular risks of exogenous testosterone use amidst men: a systematic review and meta-analysis. Am J Med. (2017) 130:293–305. doi: 10.1016/j.amjmed.2016.09.017
PubMed Abstract | CrossRef Total Text | Google Scholar
44. Cheetham TC, An J, Jacobsen SJ, Niu F, Sidney S, Quesenberry CP, et al. Clan of testosterone replacement with cardiovascular outcomes among men with androgen deficiency. JAMA Intern Med. (2017) 177:491. doi: x.1001/jamainternmed.2016.9546
PubMed Abstract | CrossRef Full Text | Google Scholar
45. Xu Fifty, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. (2013) 11:108. doi: 10.1186/1741-7015-xi-108
PubMed Abstract | CrossRef Full Text | Google Scholar
46. Liu PY, Wishart SM, Handelsman DJ. A double blind, placebo-controlled, randomised clinical trial of recombinent human chorionic gonadotropin on muscle strength and physical function and activity in older men with partial age-related androgen deficiency. J Clin Endocrinol Metab. (2002) 87:3125–35. doi: x.1210/jcem.87.seven.8630
CrossRef Full Text | Google Scholar
47. La Vignera South, Condorelli RA, Cimino L, Russo GI, Morgia K, Calogero AE. Late-onset hypogonadism: the advantages of treatent with human being chorionic gonadotropin rather than testosterone. Aging Male. (2016) 19:34–9. doi: x.3109/13685538.2015.1092021
PubMed Abstract | CrossRef Full Text | Google Scholar
48. Depenbusch G, von Eckardstein S, Simoni M, Nieschlag East. Maintenance of spermatogenesis in hypogonadotropic hypogonadal men with homo chorionic gonadotropin alone. Eur J Endocrinol. (2002) 147:617–24. doi: x.1530/eje.0.1470617
PubMed Abstract | CrossRef Full Text | Google Scholar
49. Roth MY, Lin Yard, Bay K, Amory J, Anawalt BD, Matsumoyo AM, et al. Serum INSL3 is highly correlated with intratesticular testosterone in normal men with acute, experimental gonadotropin deficiency stimulated with low-dose hCG: a randomized-controlled trial. Fertil Steril. (2013) 99:132–9. doi: 10.1016/j.fertnstert.2012.09.009
CrossRef Total Text | Google Scholar
50. Foresta C, Strapazzon Thousand, De Toni L, Perilli Fifty, Di Mambro A, Muciaccia B, et al. Bone mineral density and testicular failure: bear witness for a function of vitamin D 25- hydroxylase in human testis. J Clin Endocrinol Metab. (2011) 96:E646–52. doi: 10.1210/jc.2010-1628
PubMed Abstract | CrossRef Full Text | Google Scholar
51. Rastrelli Thousand, Carter EL, Ahern T, Finn JD, Antonio L, O'Neill TW, et al. Development of and recovery from secondary hypogonadism in aging men: prospective results from the EMAS. J Clin Endocrinol Metab. (2015) 100:3172–82. doi: 10.1210/jc.2015-1571
PubMed Abstract | CrossRef Full Text | Google Scholar
If Someone Has Hypogonadism Gonad Ism Can They Have Babies
Source: https://www.frontiersin.org/articles/10.3389/fendo.2019.00372/full
0 Response to "If Someone Has Hypogonadism Gonad Ism Can They Have Babies"
Post a Comment